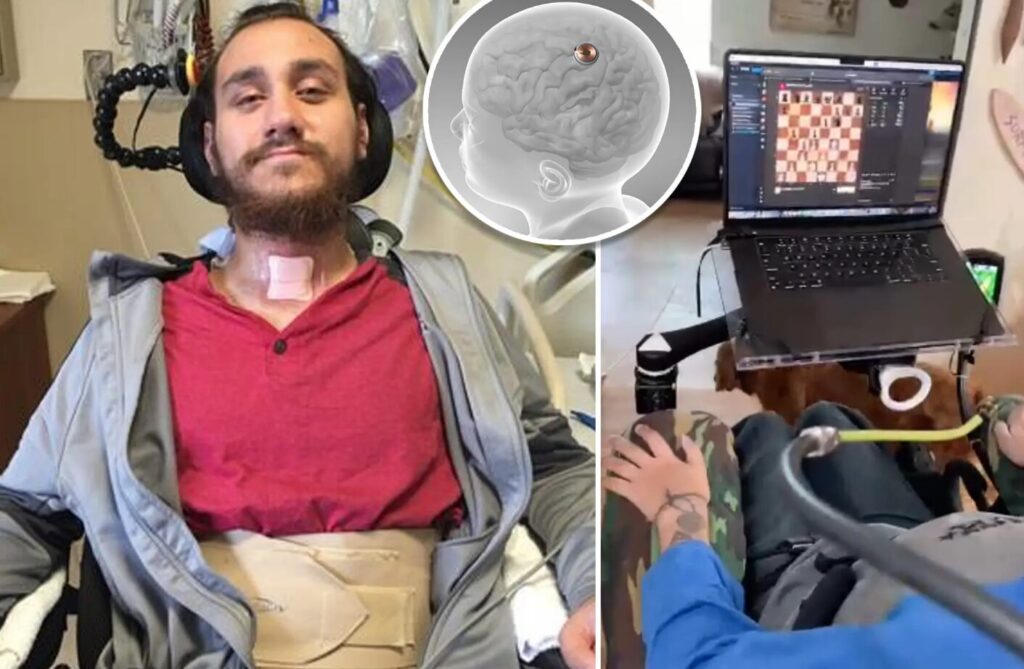
Elon Musk’s brain-computer interface company, Neuralink, has encountered a hurdle in its human trials. The first patient implanted with the company’s chip, Noland Arbaugh, experienced an issue with some of the device’s connective threads detaching from his brain.
Arbaugh, a quadriplegic since a diving accident, received the implant in January 2024 as part of Neuralink’s PRIME Study. Initial reports, including a demonstration by Musk himself, showed promise – Arbaugh could reportedly control a computer cursor with his thoughts.
However, just weeks after the surgery, Neuralink acknowledged a problem. A blog post by the company revealed that a number of the chip’s ultra-thin threads, designed to pick up brain signals, became dislodged from Arbaugh’s brain tissue. This detachment reduced the amount of data the implant could collect, impacting its effectiveness.
Neuralink Adapts: Overcoming the Challenge
While Neuralink offered limited details about the cause of the detachment, the company did announce a positive development. They were able to adjust the implant’s sensitivity to compensate for the lost data channels. This workaround apparently improved the device’s performance.
The Road Ahead: Safety, Regulation, and Long-Term Goals
Neuralink’s primary focus in this initial trial is to assess the safety of the implant and the surgical robot used for its insertion. They are also evaluating the functionality of the device itself. Regulatory approval from the Food and Drug Administration (FDA) is a critical next step before the technology can reach a wider audience.
Despite the recent setback, Neuralink remains ambitious. Their ultimate goal is to create a brain-computer interface that allows paralyzed individuals to control devices like smartphones and computers, or even restore sight to the blind.
The company envisions a future where brain implants like theirs, potentially called “Telepathy,” can empower people with disabilities to communicate and interact with the world in groundbreaking ways. However, widespread consumer access to this technology remains a long way off.
Neuralink must first navigate the complex world of regulatory approval before their brain implants become a reality for the public.